Confluent
SprayGel Pamphlet
http://www.confluentsurgical.com/spraygel/Patient%20Pamphlet%20121602.pdf
©
2001 European
Society of Human Reproduction and Embryology
Evaluation of a sprayable polyethylene glycol
adhesion barrier in a porcine efficacy model
R. Ferland1,3,D.
Mulani2 and P.K. Campbell2
1Women and Infants Hospital,
Providence, RI 02905 and 2 Confluent Surgical Inc.,
101A First Avenue, Waltham, MA 02451, USA
>Abstract
BACKGROUND: The formation of adhesions
following pelvic surgery remains one of the leading causes of infertility,
small bowel obstruction and re-operation for pelvic pain. A novel hydrophilic
polyethylene glycol based adhesion barrier (SprayGel) is formed by simultaneously
spraying two liquid precursors onto surgical sites. The liquids polymerize
to form a gel that effectively coats and adheres to tissue. After about
5 days, the hydrogel layer is absorbed and subsequently undergoes renal
clearance. It is believed that the presence of such a barrier would inhibit
the formation of adhesions following surgical insult. METHODS: A porcine
adhesion model was developed wherein bilateral uterine horn transection
and re-anastomosis, along with peritoneal side wall excision was performed
via laparotomy. In each animal (n = 10, including the pilot study) one
pelvic side wall was treated with adhesion barrier, while the contralateral
side remained untreated. RESULTS: At second look laparoscopy, 90% of the
untreated sites had adhesions, compared with 30% of the treated sites (P
= 0.006). Also observed were statistically significant reductions in the
adhesion extent (P = 0.029) and adhesion severity scores (P = 0.023) at
the treated sites. However, if the pilot study was excluded (n = 8) the
differences obtained were no longer significant. CONCLUSIONS: Polyethylene
glycol (SprayGel) merits further investigation as an effective barrier
to the formation of post-operative adhesions in this porcine model.
Key words: adhesion prevention/post-surgical adhesions/large
animal models/laparoscopy/adhesion barriers
> Introduction
Pelvic surgery often causes unavoidable tissue
injury that can lead to the formation of post-surgical adhesions in more
than 50% of patients (Trimbos-Kemper et al., 1985 ; Golan et al., 1995
). Such injuries include mechanical trauma from retractors and tissue handling,
ischaemia at suture sites and after electrocautery use, foreign bodies,
tissue desiccation and infection (Stangel et al., 1984). These stimuli
of adhesion formation occur in both open and laparoscopic approaches.
Post-operative adhesions are a common cause of
small bowel obstruction and re-operation for pelvic pain, and adhesions
involving the ovaries or Fallopian tubes are responsible for 15–20% of
female infertility cases (Ray et al., 1998 ). In addition to these adverse
clinical sequelae, the economic impact in the USA in 1994 was significant
with a direct cost of $1.33 billion for all hospitalizations during which
adhesiolysis was performed, based on Ray's analysis (Ray et al., 1998 ).
Other studies have shown that of surgical patients, 35% were readmitted
at least once for problems directly or possibly related to adhesions over
a 10 year period (Ellis et al., 1999 ; Lower et al., 2000 ).
Although the ultimate solution to this problem
will probably result from an increased understanding of the humoral agents
and cellular events that control adhesion formation, current clinical needs
could be met by an effective adhesion barrier that is easy to apply in
both open and laparoscopic procedures.
The majority of models developed to study adhesion
barriers utilize small animals, such as rats (Golan et al., 1995 ; Hellebrekers
et al., 2000 ), mice (Haney and Doty, 1992 ) and rabbits (Marana et al.,
1997 ). These models use a variety of means to mimic surgical injury, such
as abrasion or electrocautery applied to a range of organs including uterine
horns (Golan et al., 1995 ), the caecum, ovaries (Marana et al., 1997 )
and the pelvic sidewall to create a nidus for adhesion formation. These
models have contributed greatly to our knowledge of adhesion formation
and prevention. However, due to differences in scale between humans and
these models, they are limited in their ability to predict clinical success.
Two studies have previously used porcine adhesion
models (Montz et al., 1993a ; Christoforoni et al., 1996 ), and in this
study a porcine adhesion model has been developed with the intent to better
mimic conditions involved in pelvic surgery. This model involves a clinically
relevant surgical injury, in a species with organ size and weights similar
to man. This model was used to evaluate the efficacy of a new synthetic
absorbable polyethylene glycol product, SprayGelTM Adhesion Barrier (Confluent
Surgical Inc., Waltham, MA, USA)
>Materials and methods
All surgical procedures were conducted in accordance
with the regulations and approval of the Rhode Island Hospital Animal Care
and Use Committee.
Animal Preparation and Operative
Procedure
10 female virgin hogs weighing 50–75 lbs were
received and acclimatized for a minimum of 2 days prior to surgery. They
were induced with a combination of tiletamine-zolazepam (5 mg/kg), xylazine
(2 mg/kg) and atropine (0.05 mg/kg) administered i.m. Pre-operatively,
the subjects received 1 gm cefazolin i.v. Following induction of the general
anaesthetic the animals were maintained on a mix of isoflurane and oxygen
inhalation for anaesthesia for the duration of the procedure. The abdominal
region was shaved, scrubbed and draped in preparation for sterile surgery.
The celiotomy was created via a single, midline
abdominal incision from the umbilicus to the symphysis pubis. The subcutaneous
tissue and fascia were divided using electrocautery (Valley Lab Force 2,
35 watts cutting current; Valley Labs, Boulder, CO, USA). The subjects
were placed in a Trendelenberg position, and the bladder was aspirated
by cystostomy with electrocautery and wall suction or 18-gauge needle.
Dry surgical gauze, towels and retractors were used to obtain adequate
exposure to the pelvic side wall during the injury process. Both uterine
horns were sharply transected at their midpoint after coagulation with
monopolar electrocautery (25 watts coagulating current), and the transected
ends of each were then re-anastomosed (end-to-end) using two interrupted
sutures (3–0 Vicryl; Ethicon, Sommerville, NJ, USA). The parietal peritoneum
of the pelvic side wall opposed to each uterine horn was then sharply excised
from the analogue of the round ligament to the infundibulopelvic ligament
to expose an area about 5x4 cm on the pelvic sidewall. Monopolar electrocautery
(25 watts coagulating current) was used to obtain haemostasis where needed.
One subject required suture ligation of a large bleeder of the pelvic sidewall.
Following bilateral peritoneal excision and uterine horn anastomoses, both
sidewalls and uterine horns were irrigated with saline to ensure adequate
haemostasis and reduce tissue drying. Figure 1 shows an intraoperative
view of a completed peritoneal injury, just prior to uterine horn transection
and anastomosis

Figure 1. Intraoperative view of a completed
peritoneal injury, just prior to uterine horn transection and anastomosis
Thereafter, a coin toss was used to randomly assign
the left or right pelvic side wall to treatment with adhesion barrier,
which was then applied to only the assigned side wall, with no application
to the uterine horn. Following application to the assigned sidewall, the
hydrogel barrier was rinsed with saline to ensure a moist, lubricious surface.
The contralateral side received no further treatment and served as the
internal control. After appropriate treatment was performed, the laparotomy
was closed in layers with continuous braided polyester (O Ethibond; Ethicon).
After closure, the abdominal incisions were injected with local anaesthetic
(2% lidocaine) for analgesia. Animals were returned to their cages where
they received food and water ad lib and i.m. pain medications, as deemed
necessary based on their behaviour, with buprenorphine 0.01 mg/kg.
Application of adhesion barrier
The SprayGel Adhesion Barrier (Confluent Surgical)
consists of two synthetic liquid precursors that, when mixed together,
rapidly cross-link to form a solid, absorbable biocompatible hydrogel in
situ. No external energy sources are required for polymerization, which
is substantially completed within a few seconds with no heat evolution.
Both precursor solutions contain upwards of 90%
water. The first precursor solution contains a modified polyethylene glycol
(PEG) polymer with terminal electrophilic ester groups while the other
precursor solution contains PEG that has nucleophilic amine end-groups.
This second precursor solution also contains methylene blue, a colourant
that is added to the formulation to facilitate visualization of the hydrogel.
The SprayGel barrier is formulated to remain adherent to the site of application
for approximately five days. At approximately that time the barrier breaks
down by the process of hydrolysis, and the liberated water-soluble PEG
molecules (<20 KDa) are absorbed and undergo renal clearance (Yamaoka
et al., 1993* ). PEG molecules of this size have been shown to have a clearance
half-life of about 15 min in mice (Yamaoka et al., 1993*).
SprayGel has passed a complete battery of tests
including cytotoxicity, genotoxicity, haemolytic potential, sensitization
and irritation. It does not affect wound healing or potentiate infections,
and has been shown to be non-toxic at 30 times the expected human dose.
After the completion of injuries and randomization,
the surgeon applied the adhesion barrier only to the pelvic side wall injury
site assigned to the treatment group. No material was applied to the uterine
horn. Figure 2* shows a typical application at a treated site. An
air-assisted sprayer (Confluent Surgical), shown in Figure 3* , was used
to carry out the deposition. The barrier was applied to achieve a thickness
so that fine tissue structures under the barrier such as small blood vessels
or muscle fibres became difficult to visualize due to the methylene blue
colourant in the gel. This was previously established to be a thickness
of 1–2 mm. The barrier was applied to the exposed subperitoneal muscle,
and extended beyond the cut border by 2–3 cm to ensure coverage of potential
ischaemic areas. Approximately 5 ml of each precursor was needed to cover
an area of 12x8 cm. Due to the hydrophilic nature of the hydrogel barrier,
treated sidewalls were irrigated with normal saline to ensure a moist,
lubricious surface prior to closure.
Figure 2. Application of SprayGel to the
peritoneal injury site. Blue coloration identifies extent of adhesion barrier
coverage.
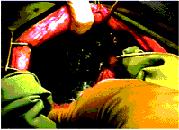
Figure 2 |
-------------------- |

Figure 3 |
Figure 3. The SprayGelTM; Adhesion Barrier System,
consisting of an applicator with two syringes containing hydrogel precursor
solutions. A small compressor (not shown) supplies air for atomization
via the connecting tubing.
Adhesion evaluation
The first two animals enrolled were evaluated
for adhesions at an earlier time point than the others (6 versus 14–16
days) to ensure adequate model response. At the time of evaluation, one
umbilical and two lower quadrant trochars were introduced. The animals
were placed in the Trendelenberg position and adhesion formation was scored
laparoscopically by a surgeon blinded to treatment site assignment. Laparoscopic
procedures were videotaped for additional evaluation if needed. The presence
or absence of adhesions at each site was noted, and extent of adhesion
coverage was scored as follows: 0, clean, no adhesions; 1, adhesions on
<50% of the stripped sidewall area; and 2, adhesions on 50–100% of the
stripped sidewall area. After this, adhesion severity was scored as: 0,
clean, no adhesions; 1, filmy adhesions; and 2, dense vascular adhesions.
Following adhesion scoring, animals were euthanized
with an i.v. potassium chloride injection, and a midline laparotomy was
performed. From this approach the laparoscopically obtained adhesion scores
could be confirmed, and representative tissues were retrieved for histological
examination. Tissues were fixed in neutral buffered formalin, embedded,
sectioned and stained using haematoxylin and eosin for light microscopy.
Statistics
To determine the statistical significance of
adhesion formation incidence between the treated and control groups, a
2 test was used. Differences in the extent and severity of adhesion formation
scores were assessed using the Wilcoxon signed rank test for nonparametric
data. All data were analyzed using the SPSS software package (SPSS Version
9.0; SPSS, Chicago, IL, USA). A value of P < 0.05 was considered to
be statistically significant
>Results
All animals survived the procedures uneventfully.
Adhesion scores for the treated and control sites of each animal, along
with the number of days implanted prior to adhesion evaluation are listed
in Table I* . At the time of the adhesion evaluation procedure, residual
adhesion barrier was not observed at any of the treated sites within the
pelvic cavity, including the two animals evaluated at 6 days. This hydrogel
barrier absorption allowed the surgeon evaluating adhesion formation to
remain blinded. This finding, coupled with the fact that the peritoneal
adhesion formation process is largely complete within 4–5 days (Holtz,
1984*, Harris et al., 1995* ), justifies the inclusion of 6 day
animal data in the larger data set.
Table I. Adhesion extent and severity scores
Pig Control sidewall
Treatment sidewall

Sidea Extent
Severity Sidea
Extent Severity
Days survived

561 L
2
1
R
0
0
6
563 R
1
2
L
0
0
6
587 L
0
0
R
0
0
16
585 L
1
2
R
0
0
16
586 R
2
2
L
1
2
16
588 L
1
1
R
0
0
16
644 L
1
1
R
2
1
14
687 R
1
1
L
1
1
16
688 L
1
1
R
0
0
16
689 L
1
2
R
0
0
16

aSidewall randomized to control or treatment (R=Right;
L=Left). |
Application of the standardized surgical injury resulted in formation
of adhesions between the uterine horn anastamosis and the pelvic sidewall
in 9 of the 10 sites randomized to control.
One animal had no adhesions to either the control or treatment sites.
Three animals had adhesions to both the control and treatment sites. No
animals had adhesions to only the treatment site. Three of the 10 sites
randomized to adhesion barrier treatment were involved in adhesions. One
of these treated sites with adhesions had required suture ligation of a
large arterial bleeder on the side wall during the initial surgery that
could not be controlled with electrocautery alone prior to randomization
to treatment. The remaining 7 of 10 treated sites were adhesion free.
When one compares the incidence of adhesion formation in the treated
and control sites, a statistically significant reduction of 67% was observed
in the treated sites (P = 0.006). Also, a statistically significant reduction
in the adhesion extent score (P = 0.029) and adhesion severity score (P
= 0.023) was observed in the treated sites. It is notable that when evaluated
without the first two (6 day) cases the differences between incidence,
extent and severity are no longer significant, probably because of the
reduced sample size (n = 8). For representative purposes, Figure 4A*
shows a gross evaluation of a treatment site without adhesions, while Figure
4B* shows a control site with adhesions.
(A) without adhesions, and a control site
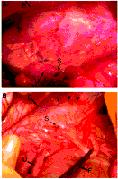
(B) with adhesions.
Reperitonealization of the treated sites was confirmed histologically
(see Figure 5A* ). Adhesions that had formed in most control sites were
vascular and some serosal thickening was evident by both gross as well
as histological analysis. A microscopic view of an adhesion free control
sidewall is shown in Figure 5B* for comparison.
Figure 5. Histological analysis of a treated site (A)
showingre-peritonealization, and of a control site (B) showing the
healed surface (from an adhesion free area). Bars = 100 µm.
(A) showingre-peritonealization, and of a control site
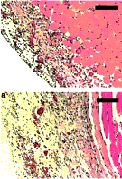
(B) showing the healed surface (from an adhesion free area).
Bars = 100 µm
> Discussion
Even though numerous adhesion barriers have been proposed and tested
both pre-clinically and clinically (Steinleiter et al., 1991*; Haney
and Doty, 1992*; Hill-West et al., 1994*; Marana et al.,
1997*; Becker et al.,1996*) there still remains a real clinical
need for an effective material to prevent site specific adhesion.
For a barrier to be clinically effective, it should be easy to use in
both laparoscopic and open procedures, as well as adhere to the desired
tissue long enough to prevent adhesions. Small animal models for adhesion
barriers typically do not allow laparoscopic device application, and organ
sizes and forces are insufficient to challenge the ability of a barrier
to remain attached to the desired site.
Many small animal models (mice, rats, rabbits) commonly described in
the literature are acceptable analogues of the biochemical processes involved
in adhesion formation. Valuable information on adhesion formation, reformation
and prevention has been obtained from these models. However, when compared
with humans, these models have differences in scale, surgical procedure,
organ size, organ weight and physiological forces. These differences can
reduce the ability of these models to predict clinical success.
To this end, some have proposed large animal models such as a canine
radical pelvic resection model that simulates radical hysterectomy (Montz
et
al., 1993b* ), a porcine incisional hernia repair model (Christoforoni
et
al., 1996* ), a porcine pelvic surgery model (Montz et al.,
1993a* ) and a ewe hysterotomy model that simulates a myomectomy (Moll
et
al., 1992* ). No large animal models have been adequately described
for the formation of adnexal adhesions with the pelvic sidewall.
In this study we present a porcine model for adhesion formation following
tubal and ovarian surgery. An attempt is made to develop site-specific
adhesions with high reproducibility using a surgical procedure analogous
to adnexal surgery or myomectomy.
This model creates typical surgical conditions encountered in tubal
and ovarian surgery, a clinically relevant procedure that is known to be
at high risk for the development of post-operative adhesions. The inclusion
of several adhesiogenic stimuli that are routine during surgery, such as
de-peritonealization of tissues and ischaemic insults from electrocautery
and sutures, creates a surgical environment optimal for evaluating adhesion
barriers. In this manner, preclinical animal model testing of proposed
prophylactic anti-adhesion materials can more accurately predict ultimate
clinical efficacy in humans. In addition to developing this animal model,
the present study also evaluated a specific adhesion barrier material,
SprayGel.
Relative to the control side, there was a 67% reduction in the incidence
of adhesions at the treated side. On the treated side, only the pelvic
sidewall received the adhesion barrier, leaving the injured uterine horn
and bladder unprotected. It is significant to note that adhesions were
observed on the treated side between the uterine horn and the bladder,
demonstrating that the barrier did indeed exclude the adhesiogenic horn
from the sidewall, but not from untreated ipsilateral sites.
High inter-animal variability in animal models has led to the suggestion
of using each animal as its own control (Ordonez et al., 1997* ).
This of course can only be performed with non-regional adhesion prevention
strategies. Since in this study the adhesion barrier is applied locally
by spraying and does not redistribute throughout the pelvis like a liquid
or viscous gel, such a model can be used resulting in distinct statistical
advantages. Moreover, the need for internal controls is particularly important
when using large animals not specifically bred for genetic similarity.
Despite the presence of several large animal adhesion formation models
in the literature, there is a need for a reliable large animal model of
adhesion formation following tubal and ovarian surgery. Given the histological
similarities between porcine uterine horns and human Fallopian tubes, this
model seems appropriate to assess post-operative adhesions following adnexal
surgery.
This porcine model also allowed the creation of conditions that are
relevant to the human surgical environment in terms of organ size and forces
exerted on the adhesion barrier. These conditions are needed to better
evaluate important barrier features such as ease of placement and resistance
to migration from the desired site.
In order to obtain consistent adhesions at the control site, it was
determined during model development that the injury process needed to be
performed via laparotomy. Therefore, the adhesion barrier was applied during
the same open procedure. In the future, laparoscopic material application
following the laparotomy closure may be performed. Even though in this
model the adhesion barrier was not deployed laparoscopically, the sprayable
nature of this barrier allows for the easy deposition in both laparoscopic
and open surgical scenarios. The presence of a colourant allows for an
easy visualization of the hydrogel and precise placement. The transformation
of the precursor solutions to a tissue adherent hydrogel takes place within
seconds and large denuded or traumatized areas can be expeditiously protected.
An undetectable amount of heat is liberated during the gelation process,
and due to the high water content of the components a lubricious surface
is presented to surrounding tissues and organs after deposition.
PEG is a poor substrate for bacteria due to its non-biological origin.
Thus, along with the rapid barrier absorption rate (less than 1 week),
the barrier material does not lend itself readily to the promotion or potentiation
of bacterial infection. Despite the emergence of several regional adhesion
prevention instillates, there is a clear need for an efficacious, easy
to use site-specific adhesion barrier that can be used laparoscopically.
Thus, SprayGel will potentially address the need of the laparoscopic surgeon
who needs to protect site-specific injuries that are susceptible to post-surgical
adhesion formation.
The results of this study lead us to conclude that this porcine model
of adhesion formation is appropriate for the investigation of site-specific
adhesion formation and prevention in a clinically relevant surgical procedure.
The promising efficacy demonstrated by the PEG adhesion barrier in this
and other (Dunn et al., 2001* ) models of adhesion formation warrant
the further investigation of this adhesion barrier material in a larger
animal study.
> Acknowledgements
The sponsors would like to thank Tom Frayne and Jim Clarke of the Rhode
Island Hospital for their expert assistance, Ogan Gurel, M.D. for his expert
critical review of this manuscript and Erin R.Campbell, Ed.D. for statistical
assistance. This work was funded with a research grant from Confluent Surgical
Inc.
> Notes
3To whom correspondence should be addressed at:
695 Eddy St., Providence, RI 02905, USA.
E-mail: roger_ferland@brown.edu
Statement of commercial interest
D.Mulani is a former employee while P.K.Campbell is a
current employee of Confluent Surgical Inc. R.Ferland is a consultant to
Confluent Surgical Inc.
> References
Becker, J.M., Dayton, M.T., Fazio, V.W. et al. (1996)
Prevention of postoperative abdominal adhesions by a sodium hyaluronate-based
bioresorbable membrane: a prospective, randomized, double-blind multicenter
study. J. Am. Coll. Surg., 183,297–306.[Medline]
Christoforoni, P.M., Kim, Y.B., Preys, Z. et al. (1996) Adhesion formation
after incisional hernia repair: a randomized porcine trial. Am. Surg.,
62, 935–938.[Medline]
Dunn, R., Lyman, M.D., Edelman, P.G. et al. (2001) Evaluation
of the SprayGel adhesion barrier in the rat cecum abrasion and rabbit uterine
horn adhesion models. Fertil. Steril., 75, 411–416.[Medline]
Ellis, H., Moran, B.J., Thompson, J.N. et al. (1999)
Adhesion-related hospital readmissions after abdominal and pelvic surgery:
a retrospective cohort study [see comments]. Lancet, 353, 1476–1480.[Medline]
Golan, A., Maymon, R., Winograd, I. et al. (1995) Prevention
of post surgical adhesion formation using aspirin in a rodent model: a
preliminary report. Hum. Reprod., 10, 1797–1800.[Abstract]
Haney, A.F. and Doty, E. (1992) Murine peritoneal injury
and de novo adhesion formation caused by oxidized-regenerated cellulose
(Interceed TC7) but not expanded polytetrafluorethylene (Gore-Tex surgical
membrane). Fertil. Steril. 57,202–208.[Medline]
Harris, E.S., Morgan, R.F. and Rodeheaver, G.T. (1995)
Analysis of the kinetics of peritoneal adhesion formation in the rat and
evaluation of potential antiadhesive agents. Surgery, 117, 663–669.[Medline]
Hellebrekers, B.W., Trimbos-Kemper, T.C., Trimbos, J.B.
et al. (2000) Use of fibrinolytic agents in the prevention of postoperative
adhesion formation. Fertil. Steril., 74, 203–212.[Medline]
Hill-West, J.L., Chowdhury, S.M., Dunn, R.C. et al. (1994)
Efficacy of a resorbable hydrogel barrier, oxidized regenerated cellulose,
and hyaluronic acid in the prevention of ovarian adhesions in a rabbit
model. Fertil. Steril., 62, 630–634.[Medline]
Holtz, G. (1984) Prevention and management of peritoneal
adhesions. Fertil. Steril., 41, 497–507.[Medline]
Lower, A.M., Hawthorn, R.J., Ellis, H. et al. (2000)
The impact of adhesions on hospital readmissions over ten years after 8849
open gynaecological operations: an assessment from the Surgical and Clinical
Adhesions Research Study. Br. J. Obstet. Gynaecol., 107, 855–862.
Marana, R., Catalano, G.F., Caruana, P. et al. (1997)
Postoperative adhesion formation and reproductive outcome using Interceed
after ovarian surgery: a randomized trial in the rabbit model.Hum. Reprod.,
12,
1935–1938.[Abstract]
Moll, H.D., Wolfe, D.F., Schumacher, J. et al. (1992)
Evaluation of sodium carboxymethylcellulose for prevention of adhesions
after trauma in ewes. Am. J. Vet. Res., 53, 1454–1456.[Medline]
Montz, F.J., Monk, B.J., Lacy, S.M. and Fowler, J.M.
(1993a) Ketorolac tromethamine, a non-steroidal anti-inflammatory drug:
ability to inhibit post-radical pelvic surgery adhesions in a porcine model.
Gynecol. Oncol., 48, 76–79.[Medline]
Montz, F.J., Monk, B.J. and Lacy, S.M. (1993b) Effectiveness
of two barriers at inhibiting post-radical pelvic surgery adhesions. Gynecol.
Oncol., 48, 247–251.[Medline]
Ordonez, J.L, Dominguez, J., Evrard, V. et al. (1997)
The effect of training and duration of surgery on adhesion formation in
the rabbit model. Hum. Reprod., 12, 2654–2657.[Abstract]
Ray, N.F., Denton, W.G., Thamer, M. et al. (1998) Abdominal
adhesiolysis: inpatient care and expenditures in the United States in 1994.
J. Am. Coll. Surg., 186, 1–9.[Medline]
Stangel, J.J., Nisbet, J.D. 2nd. and Settles, H. (1984)
Formation and prevention of postoperative abdominal adhesions. J. Reprod.
Med., 29, 143–156.[Medline]
Steinleiter, A., Lambert, H., Kazensky, C. et al. (1991)
Poloxamer 407 as an intraperitoneal barrier material for the prevention
of post-surgical adhesion reformation. Obstet. Gynaecol., 77, 48–52.[Medline]
Trimbos-Kemper, T.C., Trimbos, J.B. and van Hall, E.V.
(1985) Adhesion formation after tubal surgery: results of the eighth-day
laparoscopy in 188 patients. Fertil. Steril., 43,395–400.[Medline]
Yamaoka, T., Tabata, Y. and Ikada, Y. (1993) Distribution
and tissue uptake of poly(ethylene glycol) with different molecular weights
after intravenous administration to mice.J. Pharm. Sci., 83, 601–606.
Submitted on October 27, 2000; accepted on August 28,
2001.
Bernie and I sponsored Mary Pomroy of Canada in her first two surgeries
with Dr. Korell, Duisburg, Germany! Both procedures offered her some relief
in her symptoms, but they were still not considered a success due to the
massive amount of adhesions that reformed post surgically both times.
I also accompanied Mary to Germany on her prior two trips for surgery and
I was instrumental in getting her selected for THIS cutting edge surgery!
Bernie and I were at the 2002 Endoscopic Medical Congress in Berlin, Germany
when the success of this procedure and barrier were presented as the first
test case for Confluent! Confluent
Spraygel has gone on to make medical history and as recent as January
2004, Dr. Reich was able to validate the Confluent
Spraygel’s efficiency by performing a follow-up laporoscopy on three
ARD patients who had Spraygel used in their
adhesiolysis procedures in 2003!
All three patients were adhesion free
where the Spraygel was applied!
Confluent Spraygel WILL revolutionize
surgery as we know it today..
Thank-GOD for that!
Significant
Clinical Result in the Treatment of Adhesions with the SprayGel Adhesion
Barrier
April 24, 2002
German Case Provides Freedom from
Pain for the First Time in 22 Years for a Canadian Woman
Waltham, MA (USA), April 24, 2002: Confluent
Surgical, Inc. announced today that the SprayGel Adhesion Barrier,
their flagship product, exceeded the clinical expectations of a leading
German gynecologic surgeon in the treatment of a patient suffering from
adhesion-related pain for twenty-two years.
Matthias Korell, MD, Privat Dozent of Gynecological
Surgery, Duisburg, Germany, is recognized for his expertise in preventing
and reducing adhesions in the abdomen and pelvis that result from previous
open or laparoscopic surgical intervention. "The SprayGel Adhesion Barrier
exceeded my expectations in reducing adhesions and represents a big step
forward in treating patients with adhesion-related pain," says Dr. Korell.
"We could see a complete adhesion-free abdominal wall one week after performing
adhesiolysis." Adhesiolysis is the surgical removal of adhesions. "Having
treated this patient since July 2000 for adhesion-related pain, this is
the first and only time that significant improvement from pain has been
recognized."
The patient, a Registered Nurse and mother of
six from Calgary, Alberta (Canada), has had over twenty abdomino/pelvic
surgeries in the last twenty-two years. As a direct result of these surgeries,
she had developed adhesions. These adhesions severely impacted her lifestyle
as evidenced by her inability to lie down or raise her arms above her head
without pain. "The pain controlled my life, resulting in the loss of my
job and dramatic feelings of isolation. Other products for adhesion reduction
have been used during my past surgeries, but the pain would return in two
to three days," said the patient. Eight days after Dr. Korell’s use of
the SprayGel Adhesion Barrier, she refers to her case as "a miracle – I
feel very good with an overall sense of wellness and, for the first time
in years I have complete mobility without pain." Dr. Korell further stated,
"there is no risk of the development of severe adhesions unless she has
another traumatic surgery. While this case is of an extreme
nature, the performance of the SprayGel Adhesion Barrier supports the benefits
of the prevention and reduction of adhesions in routine gynecological surgery
that may result in adhesion formation."
Adhesions and fibrous bands of tissue between
adjacent organs of the body are typically caused by surgery and are directly
related to pain. Adhesions can start to form within three hours after surgery
and will cease forming when the sites of surgery heal, typically within
seven days following surgery. It is estimated that adhesions affect over
five million women and men worldwide and that the global market potential
for total abdominopelvic adhesion management is estimated at approximately
$2.6 billion (US).
Evidence of adhesion prevention and reduction
is discovered when a surgeon takes a second look at the surgical site through
a laparoscope, an instrument used to perform less-invasive surgery.
Amar S. Sawhney, Ph.D., founder, President and
CEO of Confluent Surgical, Inc., said, "we are very pleased with our clinical
results in Europe to date and believe that cases such as Professor Korell’s
represent additional evidence that differentiates the SprayGel Adhesion
Barrier from all other adhesion barrier products on the market today."
The SprayGel Adhesion Barrier received CE mark
in November 2001, and has been introduced in major markets in Europe. Based
on a European clinical study, the patented and proprietary synthetic material
of the SprayGel Adhesion Barrier has been shown to reduce or eliminate
adhesion formation following abdominopelvic surgery. The SprayGel Adhesion
Barrier is not commercially available in the United States.
Confluent Surgical, Inc. is a privately held company
developing products based on its platform of in-situ polymerized biomaterials
and associated delivery systems. For more information please contact Roberta
Sawyer at rsawyer@confluentsurgical.com
or visit the Confluent Surgical website at www.confluentsurgical.com.
“It takes one person to start a war, takes one
to end it. It takes one person to say "I care", and takes one not to. It
takes one person to understand, it takes one to be unkind. It takes one
to motivate a change, takes one to be negative. It takes one person to
make or break something, courage and bravery lies in someone to start a
change in the world.
Do you have what it takes? That one person could
be you and you can bring a change to this world with peace. Please
think about it.
by Kenneth Walterhouse
2001 – 2002 LEGISLATURE
LRB–3838/1
PJD:jld:kjf
2001 ASSEMBLY RESOLUTION 35
1
Relating to: urging increased awareness of Adhesion Related Disorder.
2
Whereas, efforts to increase awareness regarding health issues that affect
3
residents of the state of Wisconsin merit the support of the Wisconsin
state assembly;
4
and
5
Whereas, it is important to call attention to the serious medical condition
of
6
adhesion related disorder (ARD), which afflicts many residents of this
great state;
7
7
and
8
Whereas, adhesions are internal scars that frequently occur after surgery
and
9
can grow to connect internal organs not normally connected leading to chronic
pain,
10
recurrent bowel obstruction, and infertility; and
11
Whereas, in extreme cases, some patients have had to endure over 20
12
operations to remove adhesions because of the propensity for regrowth of
scar tissue;
13
and
14
Whereas, ARD may become so severe that patients are unable to work; health
15
care or disability insurance is difficult to obtain; family life is devastated;
and the
16
emotional health of ARD sufferers is severely affected, leading to life–threatening
17
depression and isolation; and
18
Whereas, hospital admissions for ARD number over 300,000 per year, rivaling
19
those for heart bypass, appendectomy, and other common operations, yet
the
20
awareness of this serious condition is relatively low; and
21
Whereas, the lack of awareness about adhesions and ARD means that many
22
doctors do not understand the condition and are unable to treat adhesions,
insurance
23
companies are unwilling to pay for treatment, and patients’ efforts to
get well are
24
continually frustrated; and
25
Whereas, the International Adhesion Society was formed in 1996 to promote
26
awareness about adhesions, to provide support to patients and information
to
27
medical professionals, and to encourage research into adhesions and their
28
prevention; and
29
Whereas, in recognition of the debilitating effects ARD has had on those
30
afflicted, it is incumbent upon the residents of this state to support
the courageous
31
individuals living and coping with this painful condition; now, therefore,
be it
32
Resolved by the assembly, That the members of the Wisconsin
assembly
33
hereby recognize ARD, promote awareness of this debilitating condition,
and
34
support further research leading to advancements in the treatment of this
disorder;
35
and, be it further
36
Resolved, That the assembly chief clerk shall transmit copies
of this
37
resolution to Beverly Doucette, the International Adhesion Society, and
the
38
Wisconsin Medical Society.
SprayGel
Adhesion Barrier System receives CE Mark (product certification)
Dr. Alain J.M. Audebert, "SprayGel is a breakthrough
because it can be easily applied laparoscopically and remains on the tissues
where it is applied during the critical wound healing period. I look forward
to its routine use."
Confluent Surgical, Inc. today announced that
it has received a CE Mark (product certification) allowing the commercial
distribution of its SprayGel Adhesion Barrier System within the European
Community.
The synthetic SprayGel Adhesion Barrier System
is designed to prevent or reduce the formation of adhesions or scar tissue
in the abdominal or pelvic cavity after laparoscopic or open surgical procedures.
SprayGel, is smooth water based, coating material
known as a hydrogel. Material is formed from two water-based polyethylene
glycols (PEG) that mix at the site of the injury.
At the end of the surgical procedure, the product
is easily sprayed onto tissue, forming an adherent, flexible barrier. The
barrier remains adhered to tissue during the critical healing period, and
then is safely absorbed.
The material is very inert and highly compatible
in the body. As no human or animal based products are used in SprayGel
the potential risks to patients are reduced.
Amar S. Sawhney, Ph.D., Founder, President and
CEO of Confluent Surgical said "The CE Mark approval represents a significant
achievement for the Company. SprayGel is the first of many products that
Confluent Surgical intends to commercialise to address the unmet needs
of post surgical adhesion prevention and surgical sealing."
Dr. Alain J.M. Audebert, of Bordeaux, France,
an Investigator in Confluent Surgical’s European clinical trial stated
"SprayGel is a breakthrough because it can be easily applied laparoscopically
and remains on the tissues where it is applied during the critical wound
healing period. I look forward to its routine use."
Confluent Surgical, Inc. is a privately held company
developing products based on its platform of in-situ polymerised biomaterials
and associated delivery systems.
Posted November 12th, 2001
New
adhesion barrier heads for multicenter trial
- Initial Study Included 14
Women
SAN DIEGO -- A sprayable adhesion barrier being
used in Europe performed well enough in a U.S. feasibility study to justify
a pivotal trial, Dr. D. Alan Johns said at the World Congress on
Endometriosis.
The hydrogel barrier, which is known asSprayGel,was
tested on 14 women in the United States who were undergoing laparoscopic
bilateral adnexal surgery for endometriosis or for lysis of adhesions,
said Dr. Johns of the Texas Institute of Clinical Research in Fort Worth.
Each woman served as her own control, with one
ovary randomly selected to receive the hydrogel barrier after surgery.
The barrier was not applied to the other ovary.
Videotapes taken at the initial laparoscopy and
at a second-look laparoscopy 3-6 weeks after the surgery were reviewed
by a surgeon who did not know which side had received the hydrogel product.
The barrier decreased the incidence of postoperative
adhesions by 71%, decreased the extent of adhesions by 69%, and decreased
adhesion severity by 43% on the sides sprayed with the hydrogel, compared
with the unsprayed sides. Surgeons removed any adhesions found on second-look
laparoscopy on both the treated and untreated sides.
The maker of the sprayable adhesion barrier,
Confluent Surgical Inc. of Waltham, Mass., funded the initial trial.
Confluent Surgical Inc. also is starting a multicenter
pivotal trial of the product in an effort to win Food and Drug Administration
approval for the indication of preventing development of adhesions after
the performance of laparoscopic surgery on adnexal structures.
Dr. Johns said he has no financial relationship
with the company other than his work on the study.
The experimental SprayGel
consists of two liquid polyethylene glycol solutions sprayed separately
and simultaneously through a bendable 5-mm sprayer onto the local site
of injury.
Within seconds, the two solutions mix and form
a hydrogel product consisting mainly of water. The gel is fully absorbed
within 7 days and its products excreted by the kidneys.
Blood does not interfere with the binding of
the product, in contrast to some other types of adhesion barriers, Dr.
Johns said at the meeting, which was sponsored by the World Endometriosis
Society and the American Society for Reproductive Medicine.
Coating tissue with barriers is only one step
in reducing the development of postoperative adhesions, Dr. Eric Bieber,
chief of ob.gyn. and medical director of the women's health service at
Geisinger Health System, Danville, Pa., said in an interview.
An essential strategy for reducing adhesions is
attention to good surgical techniques, including minimizing blood loss,
using the least amount of sutures and the least reactive sutures possible,
and decreasing devitalization of tissue.
In the future, surgeons may also try to modulate
the inflammatory response that helps incite adhesion formation or use tissue
plasminogen activator to help break down some of the products needed for
adhesions to form, he speculated.
"There remain significant opportunities for the
development of better anti-adhesion adjuvant therapy.
"It will probably take a multifaceted approach
to bring the risk of adhesions down as low as we can get it," Dr. Bieber
said.
Confluent
Surgical, Inc.

SprayGel is a new medical product designed to
prevent pelvic or abdominal adhesion formation following Laparoscopic or
Open surgery.
The easy-to-use applicator allows for the precise
application of a safe, adherent, flexible hydrogel adhesion barrier. The
barrier protects during the normal adhesion formation period, and then
absorbs and is excreted in the urine.
Two clinical trials, one in Europe and one in
the U.S., have evaluated the safety and efficacy of SprayGel.
|
SprayGel is not approved for sale in the
United States.
SprayGel is currently under clinical investigations
in the U.S. |

|
 |
http://www.adhesionprevention.com/spraygel1/us_news.htm

Significant Clinical Result in the Treatment of Adhesions with the
SprayGel Adhesion Barrier
German Case Provides Freedom from Pain for the First Time in 22 Years
for a Canadian Woman
Waltham, MA (USA), April 24, 2002: Confluent Surgical,
Inc. announced today that the SprayGel Adhesion Barrier, their flagship
product, exceeded the clinical expectations of a leading German gynecologic
surgeon in the treatment of a patient suffering from adhesion-related pain
for twenty-two years.
Matthias Korell, MD, Privat Dozent of Gynecological Surgery, Duisburg,
Germany, is recognized for his expertise in preventing and reducing adhesions
in the abdomen and pelvis that result from previous open or laparoscopic
surgical intervention. "The SprayGel Adhesion Barrier exceeded my expectations
in reducing adhesions and represents a big step forward in treating patients
with adhesion-related pain," says Dr. Korell. "We could see a complete
adhesion-free abdominal wall one week after performing adhesiolysis." Adhesiolysis
is the surgical removal of adhesions. "Having treated this patient since
July 2000 for adhesion-related pain, this is the first and only time that
significant improvement from pain has been recognized."
The patient, a Registered Nurse and mother of six from Calgary, Alberta
(Canada), has had over twenty abdominopelvic surgeries in the last twenty-two
years. As a direct result of these surgeries, she had developed adhesions.
These adhesions severely impacted her lifestyle as evidenced by her inability
to lie down or raise her arms above her head without pain. "The pain controlled
my life, resulting in the loss of my job and dramatic feelings of isolation.
Other products for adhesion reduction have been used during my past surgeries,
but the pain would return in two to three days," said the patient. Eight
days after Dr. Korell’s use of the SprayGel Adhesion Barrier, she refers
to her case as "a miracle – I feel very good with an overall sense of wellness
and, for the first time in years I have complete mobility without pain."
Dr. Korell further stated, "there is no risk of the development of severe
adhesions unless she has another traumatic surgery. While this case is
of an extreme nature, the performance of the SprayGel Adhesion Barrier
supports the benefits of the prevention and reduction of adhesions in routine
gynecological surgery that may result in adhesion formation."
Adhesions and fibrous bands of tissue between adjacent organs of the
body are typically caused by surgery and are directly related to pain.
Adhesions can start to form within three hours after surgery and will cease
forming when the sites of surgery heal, typically within seven days following
surgery. It is estimated that adhesions affect over five million women
and men worldwide and that the global market potential for total abdominopelvic
adhesion management is estimated at approximately $2.6 billion (US).
Evidence of adhesion prevention and reduction is discovered when a surgeon
takes a second look at the surgical site through a laparoscope, an instrument
used to perform less-invasive surgery.
Amar S. Sawhney, Ph.D., founder, President and CEO of Confluent Surgical,
Inc., said, "we are very pleased with our clinical results in Europe to
date and believe that cases such as Professor Korell’s represent additional
evidence that differentiates the SprayGel Adhesion Barrier from all other
adhesion barrier products on the market today."
The SprayGel Adhesion Barrier received CE mark in November
2001, and has been introduced in major markets in Europe. Based on a European
clinical study, the patented and proprietary synthetic material of the
SprayGel Adhesion Barrier has been shown to reduce or eliminate adhesion
formation
Confluent Surgical, Inc. is a privately held company
developing products based on its platform of in-situ polymerized biomaterials
and associated delivery systems. For more information please contact Roberta
Sawyer at rsawyer@confluentsurgical.com
or visit the Confluent Surgical website at www.confluentsurgical.com.
© 2002, Confluent Surgical, Inc. all rights reserved.
Thursday, 26 October, 2000, 07:02 GMT 08:02 UK
Gel
'heals wounds without scars'
The gel can be sprayed on during surgery
A new spray has been developed to heal wounds made inside the body during
surgery without scarring.
The spray gel forms a bright blue "sticking plaster" and stops tissues
sticking together to form painful scars.
One of the advantages of the new dressing is that it can be used during
keyhole surgery, which is now commonly preferred to open surgery techniques.
The company which has developed the gel, Confluent Surgical in Massachusetts,
says that 60 patients have so far been treated with it.
The gel is made up of two liquids which, when sprayed together, solidify
to form a bright blue material which breaks down gradually over about a
week. The pain and discomfort of internal adhesions due to surgical scarring
can often require a further operation.
The spray, made from polyethylene glycol, is now undergoing clinical
trials in Germany and France as well as the U.S.
Head of Confluent Surgical, Amarpreet Sawhney told New Scientist that
the gel may also have the potential to halt tumour growth by cutting off
its blood supply.
"We're talking about mechanical anti-angiogenesis. We can shut off every
street or alley supplying the tumour," he said.
External wounds
Vanessa Jones, senior lecturer at the Wound Healing Research Unit at
the University of Wales College of Medicine, said the gel seemed to be
using technology which is currently applied to external wounds.
"These products have been used extremely successfully to cover external
wounds to ensure moist wound healing and avoid allergic reaction," she
said.
"It would be very beneficial if the same technology could be applied
to internal wounds."
There are benefits to laparoscopic surgery in terms of reduced time
and trauma, but there have been difficulties in managing the wounds, she
added.
Sprayable
Adhesion Barrier Effective in Patients Undergoing MyomectomyCME
News Author: Laurie Barclay, MD
CME Author: Charles Vega, MD, FAAFP
To earn CME credit, read the news
brief along with the CME information that follows and answer the post test
questions.
Release Date: August 12, 2004;
Valid
for credit through August 12, 2005
Aug. 12, 2004 — The sprayable adhesion barrier
SprayGel was safe, effective, and well-tolerated in patients undergoing
myomectomy, according to the results of a randomized, prospective trial
published in the August issue of Fertility and Sterility.
"Despite meticulous microsurgical techniques and
the adoption of laparoscopic approaches to minimize insult to sensitive
tissues, the problem of adhesions persists," write Liselotte Mettler, MD,
from Christian-Albrechts-University Kiel, in Germany, and colleagues. "Although
a number of products have been evaluated to reduce or prevent adhesions,
many are difficult to deliver to the target tissue by laparoscopic approaches....
In addition, some of these products are either not absorbable or resorb
too quickly, resulting in minimal efficacy; or have actually demonstrated
increased risks of complications from adverse effects."
SprayGel is a synthetic hydrogel which forms an
absorbable, flexible, adherent gel barrier when two polyethylene glycol-based
liquids are sprayed onto target tissue. It remains intact where applied
for approximately five to seven days, protecting the target tissue during
wound healing, and then it is hydrolyzed gradually into polyethylene glycol
constituent molecules that are resorbed and rapidly cleared by the kidneys.
In this European multicenter, phase 3 trial, 66
women undergoing laparoscopic or open uterine myomectomy were enrolled
for a 15-month period and randomized to receive either optimal surgical
treatment plus adhesion barrier or optimal surgical treatment alone. Mean
age was 34.9 years (range, 23 to 52 years). Of 64 patients, 40 (62.5%)
returned for second-look laparoscopy.
When compared with initial surgery, the mean adhesion
tenacity score of adhesions seen at second-look laparoscopy was 64.7% lower
in patients receiving adhesion barrier than in control patients (0.60 vs
1.7). Compared with initial surgery, mean adhesion extent score at second-look
laparoscopy was 4.5 vs 7.2 cm2, and mean adhesion incidence score was 0.64
vs 1.22. There were no adverse effects attributed to the adhesion barrier.
Study limitations were difficulty measuring and
quantifying adhesions, lack of physician blinding in all cases at second-look
laparoscopy, and insufficient power to determine the efficacy of this adhesion
barrier in different surgical procedures and populations.
"This unique material circumvented many of the
problems associated with earlier attempts to prevent adhesions and in our
experience did not otherwise adversely affect patient health or outcomes,"
the authors write. "This product might be well suited for infertility surgery,
endometriosis, pelvic floor repair, and any surgeries carried out for adhesiolysis.
It might well have practical application in cancer surgery, given the need
for repeat procedures, but this needs to be further investigated."
Confluent Surgical, the maker of SprayGel, funded
this study.
Fertil Steril. 2004;82:398-404
Learning Objectives for This Educational
Activity
Upon completion of this activity, participants
will be able to:
· Identify symptoms and complications
associated with adhesions.
· Evaluate the benefits and efficacy of
SprayGel in the prevention of postoperative adhesions.
Clinical Context
Adhesions are one of the most common long-term
complications associated with gynecologic surgery. Multiple negative consequences,
including chronic pelvic pain, infertility, and intestinal obstruction
have been associated with the presence of adhesions. In addition, subsequent
surgery may be made more complicated because of the presence of adhesions.
To date, few intraoperative interventions have
been demonstrated to reduce the incidence of adhesions. However, Johns
and colleagues demonstrated that SprayGel, a synthetic hydrogel that forms
an immediate flexible, absorbable, and adherent barrier when sprayed on
tissue, could reduce adhesion formation by 71%. Their results, which were
published in the November 1999 issue of the Journal of the American
Association of Gynecologic Laparoscopists, also demonstrated a 69%
reduction in the extent of adhesions when comparing treatment with SprayGel
to routine intraoperative care.
Study Highlights
· Patients recruited for the study
included women at least 18 years old scheduled to undergo laparoscopic
or laparotomic myomectomy. It was considered that all patients would benefit
from second-look laparoscopy between 3 to 16 weeks after the original procedure.
· Surgeons were blinded to treatment assignment
until the end of the surgical procedure, at which time SprayGel (mixed
with diluted methylene blue to help visualization) applied to all suture
lines and other adhesiogenic surfaces or no further treatment was used.
· The main study outcome was the severity
of adhesions at second-look laparoscopy. Severity was assessed by measuring
the location and tenacity (grade 0, no adhesions; grade 3, cohesive adhesions)
of each adhesion. Mean area of the uterus covered by adhesions was also
measured.
· Adverse events were monitored. The first
two patients who consented both received SprayGel to familiarize the surgeons
with the technique.
· 64 women were randomized into the study
protocol. Approximately one third of participants refused second-look laparoscopy,
and more women in the SprayGel group returned for another laparoscopy than
the routine surgery group.
· 82.4% of the SprayGel group had their
myomectomy performed laparoscopically, which was similar to the percentage
in the routine surgery group (76.7%). Most patients had intramural fibroids
removed.
· Baseline number and severity of adhesions
were similar between the two treatment groups.
· Mean time to apply SprayGel was 3.7
minutes, and 1.9 kits were used per patient.
· 31.8% of subjects in the SprayGel group
were adhesion free at second-look laparoscopy vs 11.1% in the routine surgery
group, a nonsignificant difference. Mean area of adhesions was also similar
between groups. However, overall tenacity scores were better in the SprayGel
group.
· Examining incident adhesions discovered
between the first operation and second-look laparoscopy, SprayGel was superior
in reducing the number and severity of adhesions. However, again, the mean
area of new adhesions was similar in the SprayGel and routine surgery groups.
· No adverse events related to the use
of SprayGel were reported.
Pearls for Practice
· Adhesions following gynecologic
surgery can result in multiple deleterious effects, including chronic pelvic
pain, infertility, and intestinal obstruction.
· In the prevention of postoperative adhesions,
SprayGel forms an absorbable, flexible, adherent gel barrier that remains
intact for approximately five to seven days, protecting the target tissue
during wound healing, and hydrolyzes gradually into molecules that are
reabsorbed and rapidly cleared by the kidneys. It may reduce the number
and severity of adhesions following laparoscopic myomectomy, but total
area of adhesions is not improved with SprayGel.
Post Test
1. Which of the following is related to adhesions
after gynecologic surgery?
a. Infertility
b. Chronic pelvic pain
c. Intestinal obstruction
d. Complications related
to later surgeries
e. All of the above
2. Which of the following was significantly improved
in the SprayGel vs routine surgery groups in the current study by Mettler
and colleagues?
a. Total number
of adhesions
b. Total number of incident
adhesions
c. Mean area of adhesions
d. Severity of adhesions
e. B and D
OBGYN.net
Conference Coverage
From the 31st Annual Meeting of the
American Association of Gynecological Laparoscopists (AAGL)
A Solution to the Problem of Adhesions
Amar Sawhney, PhD interviewed by OBGYN.net
Editorial Advisory,
Professor
Liselotte Mettler, MD
November 2002
A Potential Solution to the Problem of Adhesions
Amar Sawhney, PhD interviewed by Professor Liselotte Mettler, MD
Click
here for Audio/Video Version *requires RealPlayer - free download
Prof. Liselotte Mettler, MD: Good
morning, Dr. Amar Sawhney, I am Professor Liselotte Mettler from the Department
of Obstetrics Gynecology, University of Kiel, and we have met before. I
know that you are the President of Confluent Surgical, welcome.We would
like to talk at the present time about adhesions, which is a very big problem
for women. Adhesions results after any surgery, any infection, and it brings
many patients to us as gynaecologists for a second surgery and we, so far,
do not have any good products to prevent new adhesions. So what is your
product that the company is at the present time supporting and doing trials
around the world, I understand. What is the product?
Amar Sawhney, PhD: Professor Mettler,
it is a pleasure to meet you and see you again. My company, the company
I am the President of, Confluent Surgical, is based out of Boston, Massachusetts,
specifically in Waltham, Massachusetts. We’re a small company, we’re working
on some innovative products and technologies, especially designed for the
problem of adhesion prevention and our products are currently going toward
laparoscopic prevention of adhesions for which there is no product currently
approved in the United States. Our product, SprayGel, which consists of
two liquid components that can be sprayed by a device that goes through
keyhole surgery, so it can be used laparoscopically.
Prof. Liselotte Mettler, MD: While
here at this meeting of the American Association of Gynecological Laparoscopists,
I see the doctors running to your booth.
Amar Sawhney, PhD: Yes, it is an
exciting development for them because they have not had this tool before
and they are looking forward to participating in the US in trials and in
Europe and Australia, where the product is actually commercially available
now and it’s been used in many countries, such as in Germany, in Italy,
and a few other countries in Europe. It has been used and in Australia
and New Zealand, also. In the United States currently, it is undergoing
multi-centre clinical testing. We have finished one series of clinical
testing with Dr Alan Johns and now we have some additional clinical trials
which are underway at some medical centers across the United States and
more information on Confluent SprayGel can be found on our website, at
confluentsurgical.com
or
spraygel.com.
Prof. Liselotte Mettler, MD: I
personally have used the gel for now probably 200 patients, and I’m very
happy with it. I think it’s one of the newer products, which has a future.
We have to wait and see. It will be very successful because of the studies
we’ve done, but we are optimistic and I do hope that you have a good future
with this product and we can help many women for this product adhesions,
which is a great problem and there is no cure for the present time.
Amar Sawhney, PhD: I hope so. We
are actually getting a lot of requests from patients who need this product
and some of them actually are going to Europe to get treated, unfortunately
right now because the product is not approved in the United States. Women
who are suffering from problems of adhesions, caused either due to previous
surgery or due to endometriosis and resulting in pelvic pain are, unfortunately,
coming to us seeking use of the product. Unfortunately right now, it is
under only clinical trials in the US and only available in, of course,
the clinical study; but in Europe, Australia and New Zealand, it is commercially
available and we hope to do that shortly in the United States, too.
Prof. Liselotte Mettler, MD: Thank
you very much.
Amar Sawhney, PhD: Thank you for
your kind interview. Appreciate your help.
EXCLUSIVE
TO
http://www.adhesionrelateddisorder.com
http://www.ardchat.com
News That Will Rock the Nation
Clinical Trials in the USA for
Adhesion Barrier
Waltham, MA (USA), September 23, 2004: Confluent Surgical, Inc.
Announced today, in an exclusive to,
“ARDchat” and “Adhesion Related Disorder Information Site”
that clinical trials for the
SprayGel Adhesion Barrier, their flagship product, has resumed in the
United States of America!

SprayGel is not approved for sale in the United States.
SprayGel is currently under clinical investigations in the U.S.
|
The SprayGel Adhesion Barrier received CE mark in November
2001, and has been introduced in major markets in Europe. Based on a European
clinical study, the patented and proprietary synthetic material of the
SprayGel Adhesion Barrier has been shown to reduce or eliminate adhesion
formation
Confluent Surgical, Inc. is a privately held company developing
products based on its platform of in-situ polymerized biomaterials and
associated delivery systems. For more information please contact Roberta
Sawyer at rsawyer@confluentsurgical.com
or visit the Confluent Surgical website at www.confluentsurgical.com.
© 2002, Confluent Surgical, Inc. all rights
reserved

